
Cloud-Based System,Featuring Wearable Biosensor with Chest-Based SpO2,Enables Active Patient Monitoring Across Hospital and Out-of-hospital Care Settings
Replacestime-consumingmanual spot checking with continuous,near real-time active patient monitoring
Web-based system displays multiple patient physiological data continuously,with visual alarms and alert notifications for streamlined patient care
Developed to support scalable population health management
Follows FDA 510(k) Clearance
MILPITAS,Calif.,Dec. 5,2024 -- LifeSignals,Inc. today announced that the UbiqVue 2A Multiparameter System has received EU MDR Certification,marking another significant milestone in deploying continuous wireless patient monitoring for population health management following FDA 510(k) Clearance last month. UbiqVue 2A is thus approved and CE-marked in accordance with EU regulation on medical devices,which ensures the safety and performance of medical equipment.This is especially significant because the UbiqVue 2A Multiparameter System is designed to be deployed in home,as well as hospital settings to continuously monitor patients' physiological data,replacing laborious and potentially inaccurate spot-checks.
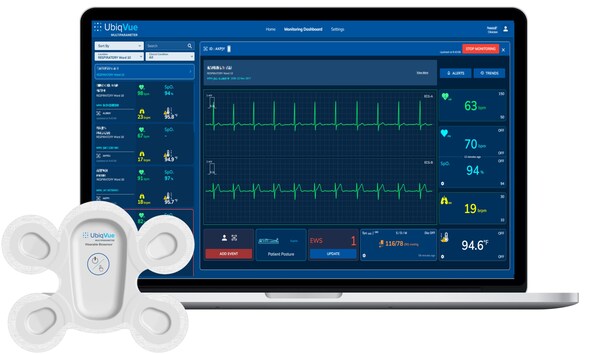
UbiqVue™ 2A Multiparameter System is based around a wearable ciosensor with chest-based SpO_2
Central to the system is the UbiqVue 2A Biosensor,a single-use,all-in-one wearable device that enables SpO2** to be continuously collected from the chest,alongside other biodata,for generating a total of twelve monitored parameters,including 2-channel ECG,pulse rate,PPG,respiration rate,body temperature,and motion. The encrypted data is securely transmitted,in near real-time,from the Biosensor via a relay app or an access point to a secure cloud-based system,where it is further signal-processed. Healthcare professionals and care providers can access continuous vital signs,via the UbiqVue web portal,and receive alert notifications.
"This Class IIb EU MDR Certification,typically given to devices intended for the continuous surveillance of vital physiological parameters in anesthesia,intensive care or emergency care,confirms our commitment to deliver bedside patient monitor-equivalent functionality through an affordable,single-use Biosensor," said Surendar Magar,Co-founder and CEO. "By securing the necessary regulatory approvals in various geographies and creating global partnerships with OEMs,service providers,and distributors,we aim to transform healthcare at scale."
The UbiqVue System is expected to play a vital role in advancing both individual patient care and broader population health strategies,reinforcing LifeSignals' mission to deliver innovative,wireless solutions for healthcare systems worldwide. Learn more by visiting: https://www.lifesignals.com/ubiqvue-multiparameter/
** White light spectral SpO2 patented technology licensed from BioIntelliSense,Inc. LifeSignals,Inc. in partnership with BioIntelliSense,carried out the product-level design and processing technology enhancements enabling reliable,continuous SpO2 monitoring and accurate performance across all skin tones in a chest-worn Biosensor.
About LifeSignals Inc.
LifeSignals delivers scalable patient monitoring solutions for population health. Our UbiqVue Wireless Patient Monitoring System features single-use,multiparameter wearable Biosensors fully driven by proprietary system-on-chip silicon technology. This ready-to-deploy solution ensures seamless,accurate,and economical patient monitoring across clinical and community settings. Learn more at www.lifesignals.com.
Disclaimer: This article is reproduced from other media. The purpose of reprinting is to convey more information. It does not mean that this website agrees with its views and is responsible for its authenticity, and does not bear any legal responsibility. All resources on this site are collected on the Internet. The purpose of sharing is for everyone's learning and reference only. If there is copyright or intellectual property infringement, please leave us a message.
©Copyright 2009-2020 Startup Weekly Contact Us SiteMap